Identify What a Coffee Cup Calorimeter Measures.
Since energy is neither created nor destroyed during a chemical reaction the heat. The coffee cups could melt.
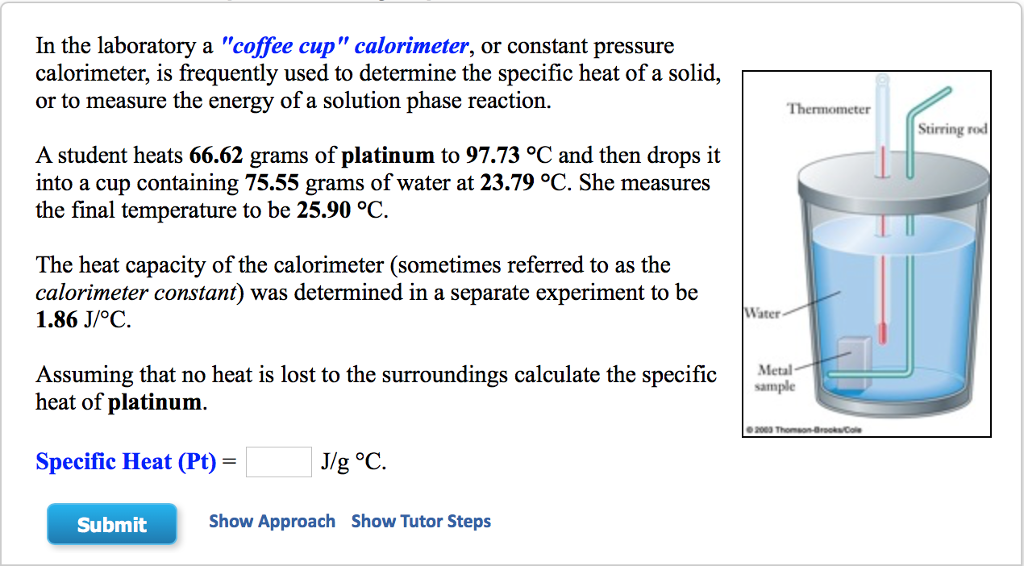
Solved In The Laboratory A Coffee Cup Calorimeter Or Chegg Com
When a 2000 mL volume of.
. 101 Choose the reaction that illustrates ΔfH for NaHCO3. B measures ΔE for combustion reactions. A coffee cup calorimeter is great for measuring heat flow in a chemical solution but it cant be used for reactions which involve gases since they would escape from the cup.
91 Two solutions initially at 2469 C are mixed in a coffee cup calorimeter Ccal 1055 J C-1. E 595 10 3 kJ. Identifying a Metal by Measuring Specific Heat.
What is the amount of heat associated with the chemical reaction if the temperature of the. First the mass of. D 168 10 3 kJ.
A coffee cup calorimeter is used to measure enthalpy changes in chemical processes giving ΔH. In the laboratory heat flow is measured in an apparatus called a calorimeter. Identifying a Metal by Measuring Specific Heat.
The cup of styrofoam with an inserted thermometer can calculate the heat transfer. A coffee cup calorimeter is a constant pressure calorimeter. A styrofoam cup makes.
4 C6H5NH2l 35 O2g 24 CO2g 14 H2Og 4 NO2g A. The outer cup is assumed to be perfectly adiabatic meaning that it does not absorb any heat whatsoever. The calorimeter is made of.
Identify what a coffee cup calorimeter measures. As such the heat that is measured in such a device is equivalent to the change in enthalpy. A calorimeter is a device used to determine heat flow during a chemical or physical change.
The reaction causes the temperature of the resulting solution to fall below room. As such the outer cup is assumed to be a perfect insulator. A chemical reaction is conducted in a coffee cup calorimeter that contains 510-4 m3 of water.
A measures ΔH for aqueous solutions. Measures Delta E for reduction reactions. In constant pressure calorimetry the calorimeter constants are used to compute the amount of heat necessary to create a certain rise in the temperature of the calorimeters contents.
Directly measure the enthalpy of magnesium. A coffee cup calorimeter will not be used to. Coffee cup is a type of insulator.
When a 300-g sample of KCl was added to 300 10 2 g of water in a coffee cup calorimeter the temperature decreased by 105 C. Measures Delta H for oxidation solutions b. The coffee cup calorimeter works as.
In the experiment a hot plate beaker graduated cylinder thermometer string copper unknown metal balance timer 2 styrofoam cups with lids are used. When a 300-g sample of KCl was added to 300 10 2 g of water in a coffee cup calorimeter the temperature decreased by 105 C. 16 Identify what a coffee cup calorimeter measures.
If the temperature rose by 329C use the information below to determine the heat capacity of the calorimeter. A coffee cup calorimeter is used to measure heat flow in solutions It is not effective for gases heat exchanges since gases can escape from the cup. Essentially the heat measured in the device is equivalent to ΔH the change in enthalpy.
Two aqueous solutions are both at room temperature and are then mixed in a coffee cup calorimeter.

Understanding Coffee Cup Calorimetry

Solved Identify What A Coffee Cup Calorimeter Measures Chegg Com

In This Lab We Will Build An Instrument Called A Coffee Cup Calorimeter Identify The List With All Brainly Com

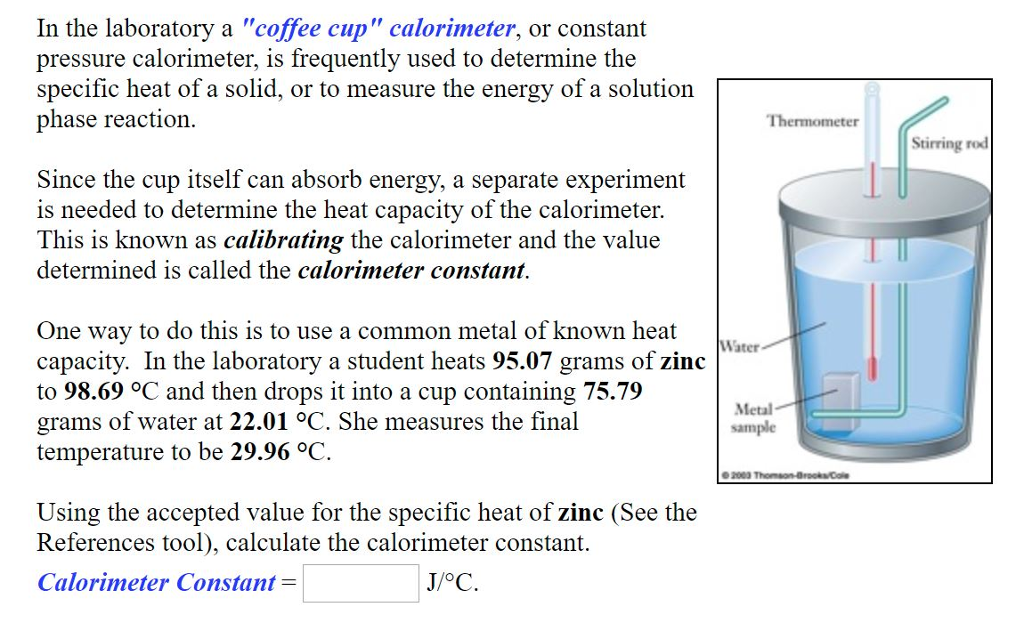
Solved In The Laboratory A Coffee Cup Calorimeter Or Chegg Com

Comments
Post a Comment